Before administering nitrous oxide therapy, you’ll need to complete several critical safety checks. Start by inspecting all gas delivery equipment, including lines, couplings, and masks for wear or damage. Verify proper ventilation system performance and calibrate monitoring devices according to manufacturer specifications. Screen patients for contraindications and guarantee appropriate PPE is ready. Document all checks and maintain continuous system monitoring. These fundamental protocols form the foundation for more thorough safety measures.
Pre-Treatment Equipment Inspection Protocols
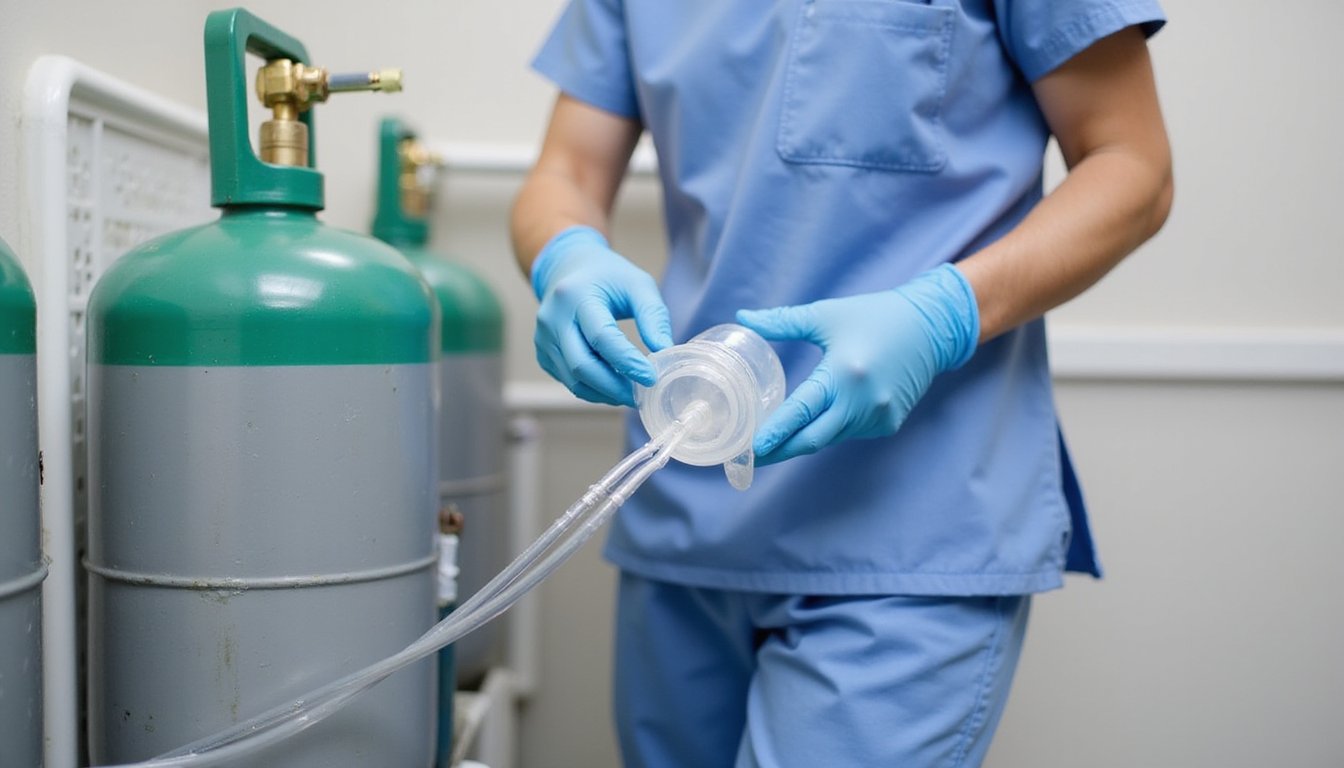
Before administering nitrous oxide therapy, thorough equipment inspection is essential to ascertain patient safety and ideal treatment outcomes. You’ll need to verify the integrity of all gas lines, checking for potential leaks using soapy water or specialized test kits. Inspect all hoses, couplings, and tubing for signs of wear, cracks, or damage. Storing gases in non-reactive containers made of steel or aluminum helps prevent contamination and degradation.
Review your cylinder storage protocols to guarantee tanks are secured upright and locked in a well-ventilated area. Test pressure regulator functionality and confirm proper operation of safety systems, including the diameter index safety system for central units or pin index safety system for portable equipment. Following manufacturer instructions for use is critical to maintain compliance with FDA regulations. Don’t forget to examine rubber masks, nasal hoods, and reservoir bags for tears or degradation. Replace single-use components immediately after each patient, and ensure all valves and connectors move freely without obstruction.
Ventilation System Performance Verification
Proper ventilation system verification builds upon thorough equipment inspection to create an extensive safety protocol. You’ll need to perform ventilation pathway mapping to guarantee compliance with NFPA 99 standards and local fire codes. System redundancy checks confirm that fail-safe mechanisms operate correctly during emergencies. Regular monitoring must include NO and NO2 concentrations using installed measurement devices to ensure patient safety.
| Verification Task | Required Action |
|---|---|
| Flow Testing | Monitor gas dispersion rates |
| Sensor Checks | Validate concentration monitors |
| Pressure Analysis | Test regulators and relief valves |
| System Integration | Verify automatic shutoffs |
Implement real-time monitoring through installed gas sensors while maintaining temperature and humidity controls in storage areas. Your verification process must include regular pressure checks, leak detection tests, and validation of emergency shutoff mechanisms. Document all test results and maintain updated ventilation system blueprints to ascertain ongoing safety compliance.
Essential Personal Protective Equipment Checks

A thorough personal protective equipment (PPE) protocol stands as your primary defense against nitrous oxide exposure risks. You’ll need pressure-demand respirators with full-face masks when N₂O levels exceed limits, along with splash-proof goggles and chemical-resistant gloves that you’ve inspected for defects.
For proper storage procedures, keep your PPE in designated contamination-free zones, away from N₂O sources. You must ensure proper sampling device placement on lapels when monitoring exposure levels near patient breathing zones. Check all equipment before use, including seal integrity tests and valve inspections. When handling cryogenic N₂O, you’ll require supplementary thermal-resistant gear and face shields for hazardous spill mitigation. Don’t forget to document all inspections in your maintenance logs and replace any compromised equipment immediately. Follow manufacturer protocols strictly, and make certain you’re maintaining compliance with OSHA standards for fit testing and training requirements.
Patient Screening and Assessment Guidelines
Before administering nitrous oxide, you’ll need to thoroughly evaluate the patient’s medical history for conditions like COPD, severe pulmonary issues, and recent head injuries that could contraindicate treatment. You must screen for allergies to nitrous oxide and oxygen, while also checking for genetic factors such as methylenetetrahydrofolate reductase deficiency that could affect metabolism. Critical contraindications include initial-trimester pregnancy and current use of medications like bleomycin sulfate, which can interact adversely with nitrous oxide therapy. The trained dental professional will carefully monitor vital signs throughout the sedation process to ensure patient safety.
Medical History Evaluation
A detailed medical history evaluation serves as the foundation for safe nitrous oxide administration. You’ll need to conduct thorough informed consent discussions and implement clear communication strategies while appraising these key areas:
| System Assessment | Required Screening | Documentation |
|---|---|---|
| Vital Signs | O2 saturation, BP, HR | Baseline values |
| Respiratory | COPD, pneumothorax | Lung capacity |
| Cognitive | Mental status, alertness | Orientation level |
| Pregnancy Status | Last menstrual period | Test results |
| Treatment History | Previous reactions | Contraindications |
You must evaluate the patient’s respiratory function through capnography monitoring and verify the absence of conditions that could compromise safety. Screen for pregnancy in females of childbearing years and document reproductive health status. Assess cognitive function to guarantee the patient can follow instructions and maintain continuous monitoring throughout the procedure.
Treatment Contraindications Assessment
Thorough screening for contraindications must precede any nitrous oxide administration to guarantee patient safety and ideal outcomes. You’ll need to identify absolute contraindications, including initial-trimester pregnancy, pneumothorax, cystic fibrosis, severe bowel obstruction, and current air embolism. Vital signs monitoring should include blood pressure, pulse rate, and oxygen saturation readings before proceeding.
Assess for respiratory and pulmonary conditions like severe bullous emphysema, COPD, and recent craniotomy. Monitor patients to obtain key reference ranges for hemoglobin and platelet counts before administration. Evaluate neurological factors, including increased intracranial pressure and impaired consciousness assessment. Check for metabolic restrictions such as vitamin B12 deficiency and methylenetetrahydrofolate reductase deficiency. Patient cooperation requirements include the ability to breathe through the nose and maintain an adequate gag reflex. Screen for substance use history and psychological impairment that could compromise treatment safety. Verify proper equipment function and room ventilation before proceeding with treatment.
Monitoring System Calibration Requirements

Proper calibration of nitrous oxide monitoring systems stands as a critical requirement for maintaining measurement accuracy and reliability. Regular calibration optimizes service life estimation and implements interference reduction techniques across your monitoring equipment. Regular sensor lifetime optimization requires scheduled maintenance and calibration to correct sensitivity drifts over time.
- Perform baseline checks using certified N₂O standards, ensuring zero and span calibrations meet manufacturer guidelines
- Maintain strict environmental controls, keeping temperature within ±3°C and pressure between 745-1015 mbar
- Recalibrate after any system transport, maintenance, or hardware modifications
- Document all calibration activities, including date, conditions, and test gas concentrations
You’ll need to verify sensor linearity across operational ranges and conduct response time checks to confirm rapid stabilization. When deviations exceed 3% or 3 ppb, immediately flag and address these variances to maintain system integrity and measurement accuracy.
Emergency Response System Readiness
A thorough emergency response system for nitrous oxide therapy requires multiple integrated safety mechanisms and precise protocols. You’ll need rapid-shutoff valves and pressure-demand respirators positioned for immediate access, with pressure-relief devices checked according to OSHA standards. Staff training procedures must include emergency simulation drills and certification in handling nitrous oxide emergencies. The scavenging system should be tested regularly to prevent occupational exposure during emergency procedures.
Your system should enable swift conversion to 100% oxygen administration when needed, with clear documentation requirements in your ePCR. Ascertain that your team can recognize the 2% oxygen saturation drop threshold that triggers immediate discontinuation. Post-incident review protocols must include detailed documentation of cylinder pressures, oxygen saturation levels, and all emergency response actions taken. Keep evacuation procedures posted and maintain regular equipment checks to guarantee system readiness.
Gas Delivery System Safety Validation
Begin your day by conducting thorough leak tests on all N₂O equipment connections, including hoses, fittings, and cylinder valves, to safeguard system integrity. You’ll need to verify pressure gauge accuracy through calibration checks against certified reference standards, documenting any deviations that exceed acceptable thresholds. Confirm proper flow control operation by testing delivery rates at multiple settings and validating that safety shutoffs respond within specified parameters. All validation procedures must be documented in the maintenance logs as per safety standards.
Daily Equipment Leak Tests
Daily leak testing of nitrous oxide delivery systems requires systematic pressure monitoring and validation to guarantee patient safety and system integrity. You’ll need to implement leak trend monitoring through regular pressure checks and periodic system audits to detect potential issues early. Regular testing must exceed what is outlined in Australian Standard 2896:2021 since routine inspections often miss significant leaks. Using nitrogen gas testing provides a safe and inert method for validating system integrity before first use.
- Monitor pressure readings at 15-minute intervals during isolation tests, watching for concerning drops that exceed 20%
- Conduct zero-demand testing during non-operational hours using alternative delivery methods
- Weigh cylinders before and after use to identify discrepancies in consumption rates
- Document all pressure data systematically to establish baseline performance metrics
For smaller systems around 3L capacity, you can detect leaks within 30 minutes, while larger systems require extended testing periods. Always maintain consistent ambient temperatures during testing to prevent false readings from thermal fluctuations.
Pressure Gauge Calibration Checks
Regular pressure gauge calibration checks form the cornerstone of gas delivery system safety validation. You’ll need to verify output stability after purging 2-3 cell volumes and examine at least three calibration points daily. Guarantee your calibration interval alignment matches manufacturer specifications while maintaining NIST standard traceability.
Monitor sensor response time carefully to avoid premature readings, and don’t proceed until measurements stabilize. You must validate your calibration graphs by confirming meter deflection matching and maintain relative standard deviations of 0.006 or less. For high-precision systems, exclude any devices showing relative uncertainties above 1×10⁻⁴. Remember to document all calibration values, including error margins, and preserve sensor response curves for your records. Cross-validate results using certified N₂O gas mixtures when possible.
Flow Control Verification
Flow control verification extends beyond basic calibration to encompass thorough safety protocols for N₂O delivery systems. You’ll need to validate both flow rate accuracy and flow rate stability to guarantee consistent therapeutic gas delivery while preventing adverse events. Digital systems display real-time flow rates and concentration levels for precise monitoring during procedures.
- Verify your gas flow output matches patient inhalation requirements to prevent breathing bag collapse and maintain optimal gas mixture
- Test the correlation between set parameters and actual delivery rates, addressing any discrepancies immediately
- Monitor scavenging suction levels to prevent premature gas removal that could affect therapeutic concentrations
- Adjust delivery rates in 10% increments while maintaining proper patient-to-technician ratios for safety
Regular flow control verification helps maintain precise gas delivery, assures reliable alarm functionality, and supports rapid emergency response capabilities. You must document all checks and adjustments to maintain compliance with safety standards.
Recovery Room Environment Standards
Maintaining strict environmental standards in nitrous oxide recovery rooms is essential for patient safety and staff protection. Your recovery area must maintain at least 15 air exchanges per hour to guarantee proper ambient gas dispersion reduction. You’ll need to implement staff exposure monitoring protocols to keep N₂O levels below NIOSH’s recommended 25 ppm TWA limit. Certified auxiliary staff must be present during the recovery phase to assist with monitoring. Keep recovery room doors closed during N₂O administration and guarantee your scavenging system maintains a 45 lpm exhaust flow. Don’t forget to use gas analyzers in high-risk areas to monitor ambient N₂O concentrations. You must also guarantee proper ventilation and avoid using N₂O in confined spaces. Document all environmental monitoring results according to your facility’s policies, and regularly inspect your exhaust systems for ideal performance and leak prevention.
Documentation and Compliance Verification
Proper documentation of nitrous oxide therapy requires strict adherence to multiple regulatory standards and clinical protocols. You’ll need to maintain detailed records that demonstrate your compliance with safety protocols and clinical guidelines. A thorough preoperative checklist must be completed and maintained in the patient’s records before administering any nitrous oxide sedation.
Maintaining thorough documentation for nitrous oxide therapy ensures regulatory compliance and patient safety through detailed record-keeping and protocol adherence.
- Obtain and file informed consent documentation before treatment, clearly outlining risks, benefits, and contraindications
- Record detailed clinical notes, including gas flow rates, concentrations, exposure duration, and patient behavioral responses
- Document regular scavenging system testing results, room ventilation rates, and negative pressure verification
- Keep records of post-procedure monitoring, including any adverse reactions and equipment safety checks
Ensure all documentation meets state-specific regulations and remains audit-ready. You must include appropriate ICD-10 codes for qualifying conditions and maintain records for the mandated retention period. Regular verification of your documentation system helps maintain compliance and patient safety standards.
Frequently Asked Questions
How Long Should Staff Wait Between Consecutive Nitrous Oxide Administration Sessions?
You’ll need to wait until complete desaturation occurs between nitrous oxide sessions. During this interval, guarantee you’ve performed ventilation system maintenance and scavenging equipment inspection. For ideal safety, you should allow 3-5 minutes of oxygen flushing post-treatment, then confirm the patient’s return to baseline consciousness. Don’t initiate new sessions until you’ve verified full recovery and proper equipment function through your standard protocols.
What Is the Maximum Duration Recommended for Continuous Nitrous Oxide Therapy?
You shouldn’t exceed 30 minutes for a single continuous nitrous oxide therapy session, with a maximum of two sessions allowed within a 24-hour period. When administering therapy, you’ll need suitable ventilation requirements and proper personal protective equipment in place. You must also guarantee your total exposure doesn’t surpass 4 hours over a 7-day period. Remember to monitor alveolar gas concentrations and maintain nitrous oxide levels at ≤50%.
Can Pregnant Healthcare Workers Safely Administer Nitrous Oxide to Patients?
You’ll need to exercise significant caution if you’re pregnant and administering nitrous oxide. While you can technically perform these duties, workplace safety considerations strongly suggest limiting or avoiding exposure due to potential fetal development risks. You should discuss your situation with your employer, as they’re required to offer alternative duties. If you must continue, guarantee you’re using proper scavenging systems and following strict exposure control protocols.
How Often Should Staff Undergo Blood Testing for Nitrous Oxide Exposure?
You’ll need regular exposure monitoring based on your workplace risk level. While there’s no universal consensus on testing frequency, you should undergo blood testing every 6 months if you’re routinely working with N2O. If your urinary N2O exceeds 120 µg/L, you’ll require more frequent health monitoring. For high-risk groups like pregnant staff or those with frequent exposure, you should increase testing to quarterly intervals and track both blood and urinary N2O levels.
What Temperature Range Is Optimal for Nitrous Oxide Storage Facilities?
You’ll need to maintain your nitrous oxide storage facilities between 70-75°F (21-24°C), ensuring minimal temperature fluctuations. Your storage area requires adequate ventilation and should be kept away from direct sunlight or heat sources. It is crucial to implement proper storage monitoring through daily temperature checks and documentation. Never allow temperatures to exceed 85°F (29°C) or drop below 40°F (4°C), as this can affect cylinder pressure and gas stability.