Several FDA-approved medications offer alternatives to Suboxone for treating opioid addiction. You’ll find options like methadone, which requires daily clinic visits, and naltrexone, available as a monthly injection or daily pill. Extended-release buprenorphine formulations like Sublocade and Brixadi provide monthly treatment with simplified protocols. Each medication has distinct advantages and safety considerations that you’ll need to evaluate with your healthcare provider to determine the most appropriate choice for your recovery expedition.
Understanding Suboxone’s Role in Medication-Assisted Treatment

While opioid addiction presents significant challenges, Suboxone has emerged as a cornerstone of Medication-Assisted Treatment (MAT) by combining buprenorphine and naloxone in a carefully formulated solution. This medication works by managing withdrawal symptoms and reducing cravings through buprenorphine’s partial activation of opioid receptors, while naloxone deters misuse. Buprenorphine’s ceiling effect makes it a safer alternative to full opioid agonists. The recommended daily maintenance dose of 16 mg/4 mg helps patients achieve optimal therapeutic benefits.
You’ll find that successful treatment requires patient compliance with sublingual administration and treatment adherence to behavioral therapy components. The long-term recovery outcomes improve significantly when patients pair Suboxone with counseling and support groups. The medication’s unique formulation helps prevent relapse by blocking the effects of stronger opioids like heroin or fentanyl.
When properly monitored, Suboxone’s lower risk of respiratory depression and prolonged therapeutic effects make it an effective option for stabilizing patients during detox and early recovery phases. Regular supervision and careful dosing cultivate ideal outcomes while minimizing dependency risks.
Key Differences Between Buprenorphine Formulations

The diverse buprenorphine formulations offer distinct delivery methods, from Suboxone’s sublingual films to Bunavail’s buccal films and Butrans’ transdermal patches.
You’ll find that formulations containing naloxone, such as Suboxone and Zubsolv, provide amplified safety profiles by deterring injection abuse through withdrawal precipitation.
Each formulation’s unique pharmacokinetic profile impacts its onset of action, with sublingual forms reaching peak plasma levels in 1.3-1.8 hours, while buccal films take 2.5-3 hours to achieve maximum concentration.
Delivery Methods and Administration
Medical professionals can choose from multiple buprenorphine formulations, each offering distinct advantages for addiction treatment. Understanding the delivery methods helps determine the most suitable option for your patients while considering sublingual administration challenges and patient self-monitoring considerations. These medications require careful neurotransmitter regulation to achieve therapeutic effects.
Both medications have moderate abuse potential which impacts how physicians prescribe and monitor these formulations.
Sublingual formulations like Suboxone and Subutex dissolve under the tongue, providing rapid absorption but requiring proper placement and consistent daily administration. These medications are designed to prevent withdrawal symptoms while treating opioid dependence.
Transdermal options (Butrans) offer weekly dosing at 5-20 mcg/hr, eliminating daily administration concerns.
Injectable Sublocade provides monthly dosing through subcutaneous administration, while Probuphine implants last six months.
Buccal films (Belbuca) dissolve in 30-60 seconds against the cheek lining, with doses ranging from 75-900 mcg.
The administration method you’ll choose depends on patient compliance needs, misuse risk, and required dosing flexibility.
Safety Profile Comparisons
Understanding safety profiles across buprenorphine formulations helps clinicians select ideal treatment options for each patient. You’ll find varying degrees of overdose risk mitigation and withdrawal management considerations across different formulations.
| Formulation Type | Safety Considerations |
|---|---|
| IV/IM (Buprenex) | Ceiling effect on respiratory depression; thrombophlebitis risk |
| Sublingual (Subutex) | Lower respiratory depression vs full agonists; oral irritation possible |
| Transdermal (Butrans) | Slow onset reduces acute overdose risk; skin reactions may occur |
| Buccal (Belbuca) | Higher bioavailability requires careful monitoring; transient dysphagia risk |
| Combo Products | Naloxone deters misuse but may precipitate withdrawal if injected |
The inclusion of naloxone in combination products provides supplementary safety features for overdose prevention, though you’ll need to carefully consider hepatic function and concurrent substance use when selecting formulations.
Extended-Release Options for Opioid Use Disorder
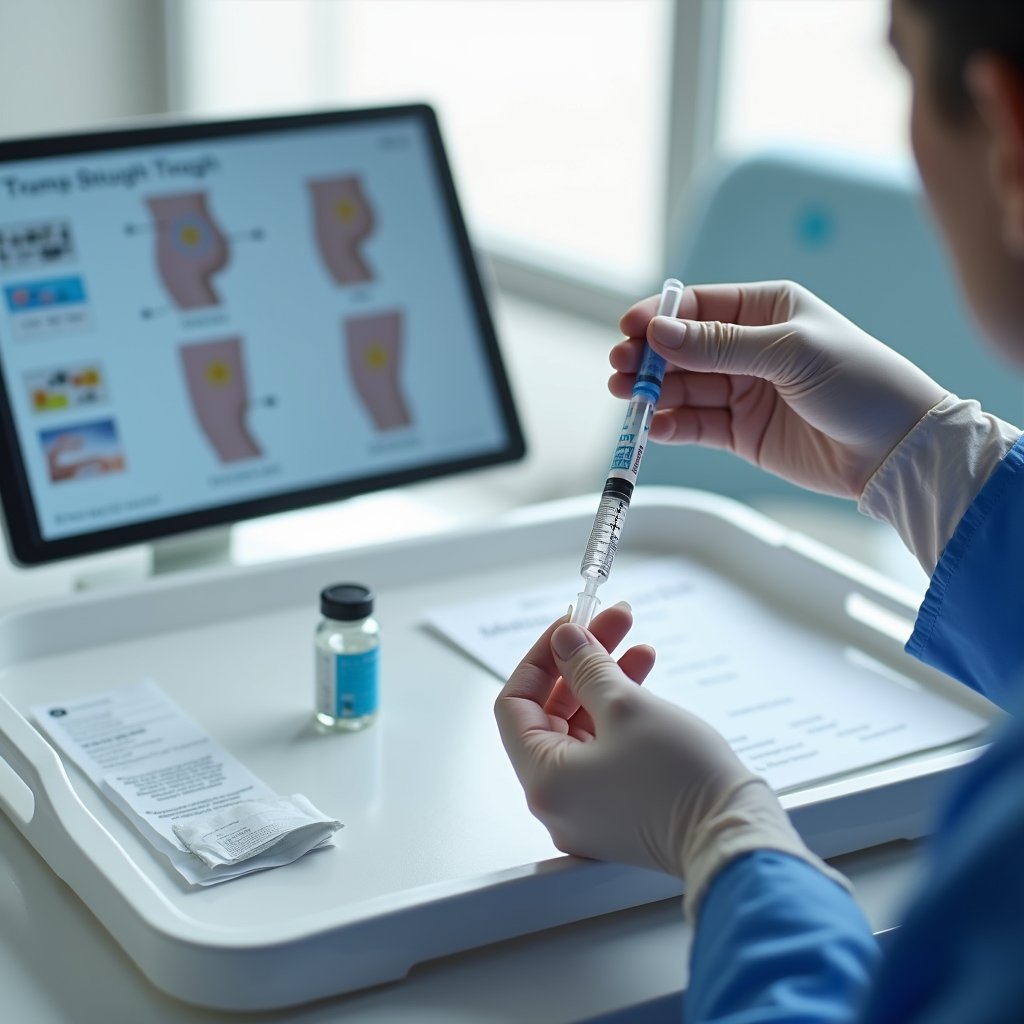
Extended-release buprenorphine injections, including Brixadi and Sublocade, offer you flexible monthly maintenance options that substantially improve treatment adherence compared to daily oral medications.
You’ll find these new delivery technologies provide consistent medication levels through subcutaneous administration in different injection sites, including the thigh, buttock, and upper arm. Both medications combine medication with counseling for comprehensive addiction treatment. These long-acting treatment options reduce your dosing frequency while maintaining therapeutic drug levels, with clinical evidence showing they cut total mortality risk by half compared to no medication treatment. Clinical studies demonstrate that patients experienced significant opioid abstinence with 300 mg maintenance doses. The rapid initiation protocol allows patients to start treatment after just a single dose of transmucosal buprenorphine followed by a brief observation period.
Monthly Injectable Benefits
As clinicians seek more effective treatment options for opioid use disorder, monthly injectable formulations like Sublocade and Brixadi have emerged as game-changing alternatives to daily oral medications. These long-acting options offer remarkable dosing flexibility while addressing key treatment barriers. Some patients transition from pure buprenorphine options like Subutex to injectable forms.
You’ll benefit from healthcare-administered injections that form a depot, ensuring gradual medication release and stable buprenorphine levels. The medication’s unique formulation blocks opioid rewards throughout the full treatment period. You won’t need to manage daily pills, which reduces stigma and eliminates common adherence challenges. Treatment plans should include counseling and support services for comprehensive care. You’ll maintain steady medication levels between doses, improving craving control and reducing withdrawal risks. You’ll have options between weekly (Brixadi 8-32mg) or monthly (Brixadi 64-128mg, Sublocade 300mg) doses, allowing for personalized treatment plans.
These injectable formulations transform addiction treatment by combining clinical effectiveness with patient-centered convenience.
New Delivery Technologies
The evolution of extended-release buprenorphine formulations marks a significant advancement in opioid use disorder (OUD) treatment. Sublocade, an injectable form of buprenorphine, now offers streamlined prescribing protocols with expanded administration sites including the abdomen, thigh, buttock, and upper arm. You’ll need only one sublingual dose and a one-hour safety check to begin treatment. A recent study showed that more than 66% of patients receiving the fast-start protocol successfully continued with their second injection.
FDA’s regulatory hurdles addressed include simplified initiation requirements and expanded delivery options. While methadone remains limited to daily formulations, the focus on extended-release technologies helps overcome adherence challenges by reducing treatment frequency. The FDA continues to prioritize expanding medication access through new formulations while maintaining safety standards. These innovations reflect ongoing efforts to improve treatment accessibility while ensuring proper medication management and patient safety.
Long-Acting Treatment Options
Several long-acting treatment options have emerged as alternatives to daily Suboxone administration for opioid use disorder (OUD). These extended-release formulations address patient preferences for reduced dosing frequency while minimizing stigma factors associated with daily medication visits.
Studies show that 40% of patients prefer injectable long-acting medication formulations for treating opioid use disorder.
Brixadi offers flexible weekly dosing (8-32mg) or monthly dosing (64-128mg), administered by healthcare providers under REMS requirements.
Sublocade provides monthly buprenorphine injections with recent label changes allowing rapid initiation and alternative injection sites.
Methadone, while administered daily, maintains long-acting therapeutic effects through specialized clinics.
Naltrexone presents a non-narcotic option that blocks opioid effects.
These long-acting alternatives strengthen treatment adherence by reducing the burden of daily dosing while maintaining therapeutic effectiveness. Your healthcare provider can help determine which option best suits your specific needs and circumstances.
Comparing Safety Profiles of Available Treatments
Modern addiction treatment options present distinct safety considerations that healthcare providers must carefully evaluate when selecting appropriate therapies. Among FDA-approved medications, Suboxone offers a superior safety profile due to its ceiling effect on respiratory depression and built-in abuse deterrent properties. When compared to methadone, Suboxone demonstrates lower metabolic risks and eliminates the need for QT interval monitoring, making it a preferred choice for medication assisted detoxification.
While naltrexone provides a non-addictive alternative that’s motivating long term recovery, it requires complete detoxification before initiation. You’ll find Suboxone’s office-based prescribing flexibility and documented 50% mortality reduction particularly compelling. The buprenorphine-naloxone combination effectively deters injection misuse while maintaining therapeutic benefits, positioning it as a cornerstone of modern addiction treatment protocols.
Benefits and Limitations of Alternative Medications
While Suboxone remains a leading medication for opioid use disorder, healthcare providers can choose from several FDA-approved alternatives that offer distinct therapeutic advantages and constraints. When selecting treatment options, providers must consider patient preference and individual clinical factors alongside medication characteristics.
Methadone offers powerful opioid blockade but requires daily clinic visits and strict monitoring protocols. Injectable naltrexone eliminates daily dosing concerns but requires complete opioid detoxification before initiation. Extended-release buprenorphine formulations provide monthly dosing convenience but may have limited availability. Non-pharmacological approaches can complement medication therapy but shouldn’t replace FDA-approved treatments.
Understanding these alternatives helps providers tailor treatment plans to each patient’s needs, considering factors like medication access, lifestyle requirements, and treatment goals. The choice between options often depends on individual circumstances and treatment history.
Accessing Treatment Through Healthcare Providers
Accessing medication-assisted treatment for opioid use disorder requires traversing specific provider requirements and clinical protocols. You’ll need to locate a healthcare provider with appropriate DEA waivers for buprenorphine prescriptions or connect with specialized opioid treatment programs for methadone. Provider training requirements vary by medication type. Generic versions of these medications can help reduce costs, with lower cost alternatives available for Suboxone and other treatment options. The Recovery Village provides comprehensive treatment plans that combine medication with behavioral therapy and counseling.
| Treatment Option | Provider Requirements | Access Points |
|---|---|---|
| Suboxone/Zubsolv | DEA Waiver | Primary Care, Telehealth |
| Methadone | OTP Certification | Specialized Clinics Only |
| Injectable MOUD | Special Training | Infusion Centers |
Clinic accessibility challenges differ based on your location and chosen medication. While traditional clinics offer in-person care, telehealth services now expand treatment access for buprenorphine medications. Mobile clinics and community health centers increasingly bridge gaps for underserved populations, though methadone still requires daily clinic visits initially.
Insurance Coverage and Cost Considerations
Understanding insurance coverage for Suboxone treatment involves traversing multiple layers of health plans and benefits. Your coverage options depend on provider qualifications and regulatory oversight requirements, which vary by state and insurance carrier. Most major private insurers and public programs cover FDA-approved medications for opioid use disorder, though specific coverage details and restrictions differ.
Prior authorization and network restrictions may apply, requiring documentation from qualified providers. Medicaid expansion and Medicare Part D improve access, but often require specific provider certifications. Manufacturer assistance programs and sliding scale clinics help reduce out-of-pocket expenses. Your total costs depend on prescription duration, provider fees, and whether you’re combining medication with counseling services.
You’ll need to verify coverage directly with your insurance carrier, as formulary placement and cost-sharing arrangements vary considerably between plans.
Latest Developments in Addiction Medicine
Recent advances in addiction medicine have transformed the scenery of opioid use disorder (OUD) treatment, introducing novel formulations and delivery methods. You’ll find emerging non-opioid therapies like Journavx (suzetrigine) targeting Nav1.8 sodium channels, reducing addiction risks in pain management. Efficiency in OUD treatment initiation has improved with Sublocade’s simplified protocols, allowing treatment starts after just one sublingual dose.
| Innovation | Clinical Impact |
|---|---|
| Sublocade | Single-dose initiation with 1-hour monitoring |
| Journavx | Inaugural non-opioid for moderate-severe pain |
| Extended-Release | Monthly dosing improves adherence |
| Flexible Injection | Multiple administration sites |
| Nav1.8 Inhibitors | Pain control without addiction risk |
These developments are reshaping treatment approaches, offering more flexible options while prioritizing patient safety and compliance through extended-release formulations and non-opioid alternatives.
Making an Informed Treatment Choice
When selecting medication for opioid use disorder (MOUD), patients and clinicians must weigh several evidence-based options against individual circumstances. Successful patient engagement and caregiver involvement strengthen treatment outcomes when choosing between Suboxone, methadone, or naltrexone-based therapies.
Choosing the right medication for opioid treatment requires careful consideration of options, with patient participation and caregiver support vital for success.
Consider your withdrawal severity and daily schedule – Brixadi’s weekly injections might work better than daily methadone clinic visits
Evaluate your support system’s capacity to assist with treatment adherence and monitoring
Review your medical history, especially respiratory or cardiac conditions that could affect medication compatibility
Assess your insurance coverage and ability to access DEA-certified providers in your area
Frequently Asked Questions
Can I Travel Internationally While Taking Suboxone or Similar Medications?
You can travel internationally with Suboxone, but you’ll need to plan carefully. Initially, check your destination country’s regulations, as some nations strictly prohibit opioid medications.
Make sure to bring an adequate supply in original packaging, along with your prescription and a doctor’s letter. You should notify travel destinations through their embassies before departure.
Always keep medications in your carry-on luggage and maintain proper documentation throughout your excursion.
How Long Does It Take to Completely Taper off Addiction Medications?
Your withdrawal timeline and medication tapering schedule will vary markedly based on several factors. You’ll typically need 4 weeks to several months for a safe taper, depending on your specific medication.
For opioid medications, you can expect a 5-10% weekly dose reduction, while benzodiazepines may require months of gradual tapering. Your healthcare provider will adjust your schedule based on your baseline dosage, withdrawal symptoms, and general health status.
Will Taking These Medications Affect My Ability to Get Life Insurance?
Taking addiction medications can impact your ability to qualify for life insurance policies, but it doesn’t automatically disqualify you. Insurance premiums may be higher initially, but showing consistent treatment adherence and stability in recovery can improve your options.
You’ll need to disclose your medication use and treatment history during the application process. Many insurers now recognize medication-assisted treatment as responsible health management, especially with documented long-term compliance and sobriety.
Can I Breastfeed While Using Medication-Assisted Treatment for Addiction?
Yes, you can safely breastfeed while on medication-assisted treatment. Research shows breastfeeding actually benefits both your postpartum mental health and your nursing infant’s safety. Your baby will have less severe withdrawal symptoms and shorter hospital stays compared to formula feeding.
While both methadone and buprenorphine transfer into breast milk at low levels, buprenorphine is often preferred due to lower concentration. Always discuss your specific situation with your healthcare provider for personalized guidance.
Do Addiction Medications Show up on Standard Pre-Employment Drug Tests?
Your medication detection time varies by drug test type and insurance policy requirements. Standard pre-employment screens don’t typically detect buprenorphine-based medications (like Suboxone), but methadone will show up on specific opiate panels.
Naltrexone isn’t detected on routine tests. You’ll want to inform your testing facility about prescribed addiction medications and provide documentation from your healthcare provider to avoid any misunderstandings during the screening process.